The Unseen Forces: An Introduction to Diving Physics
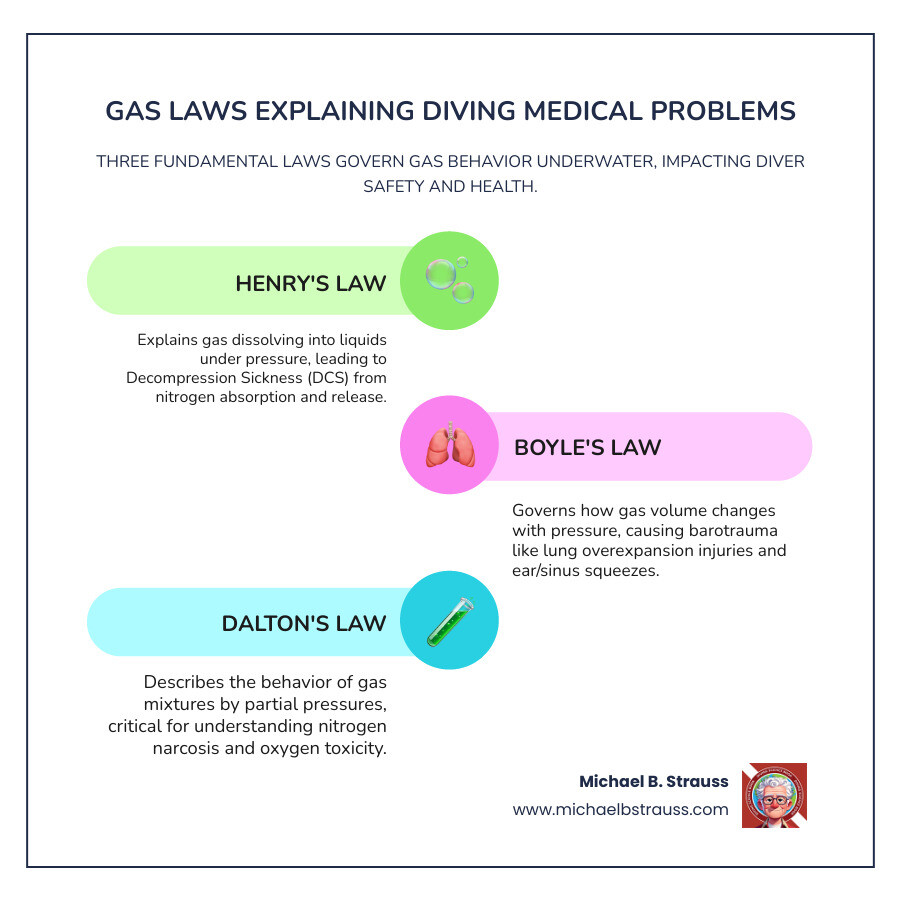
Which gas law best explains diving medical problems comes down to three main laws working together, but Henry's Law takes the lead role. Here's the quick answer:
Primary Gas Laws Explaining Diving Medical Problems:
- Henry's Law - Explains decompression sickness (DCS) and nitrogen absorption
- Boyle's Law - Causes barotrauma and lung overexpansion injuries
- Dalton's Law - Creates nitrogen narcosis and oxygen toxicity through partial pressures
With over 10 million divers worldwide, understanding these physics principles isn't just academic - it's life-saving knowledge. Every time you descend underwater, invisible forces start working on your body. Pressure increases by one atmosphere for every 33 feet of seawater, fundamentally changing how gases behave in your blood and tissues.
Think of opening a shaken soda bottle. The fizzing bubbles you see happen because dissolved gas comes out of solution when pressure drops. Your body works the same way underwater.
As Dr. Michael B. Strauss explains in his diving medicine research, these gas laws govern everything from why you can't hold your breath during ascent to why deep divers sometimes act drunk from nitrogen narcosis.
The physics might seem complex, but the core concept is simple: gases dissolve into liquids under pressure and come back out when pressure decreases. Understanding this relationship helps prevent serious medical emergencies that can happen when divers ascend too quickly or dive beyond safe limits.
Which gas law best explains diving medical problems word roundup:
Which Gas Law Best Explains Diving Medical Problems?
Picture yourself descending into the blue depths, and invisible forces immediately start working on your body. Which gas law best explains diving medical problems isn't just one answer - it's three powerful laws working together like a perfectly choreographed underwater ballet.
Think of it this way: every breath you take underwater sets off a chain reaction of physics that can either keep you safe or put you in serious danger. The difference lies in understanding how these laws affect your body and respecting their power.
Henry's Law: The Primary Answer to Which Gas Law Best Explains Diving Medical Problems
When people ask which gas law best explains diving medical problems, Henry's Law usually steals the spotlight - and for good reason. It's the mastermind behind decompression sickness, also known as "the bends."
Here's the simple version: Henry's Law says that gases dissolve into liquids under pressure. More pressure means more gas gets absorbed. Since your body is mostly water, this law directly affects you every time you dive.

Remember opening a shaken soda bottle? The fizzing happens because dissolved carbon dioxide rushes out when pressure drops. Your body works exactly the same way with nitrogen.
As you descend, pressure increases and more nitrogen dissolves into your blood and tissues through a process called on-gassing. The deeper you go and longer you stay, the more nitrogen gets packed away in your system. It's like your tissues become tiny nitrogen storage tanks.
The trouble starts when you ascend. As pressure decreases, that dissolved nitrogen wants to come back out through off-gassing. If you rise too quickly, nitrogen can't escape through your lungs fast enough. This creates supersaturation - more dissolved gas than your body can handle at that pressure.
Just like that soda bottle, bubbles start forming in your blood and tissues. These bubbles cause decompression sickness (DCS), creating symptoms ranging from joint pain to paralysis or even death. The bubbles can lodge anywhere - your joints, spinal cord, or brain - which explains why DCS symptoms vary so dramatically.
This is why slow ascents and proper decompression stops are absolutely critical. Your body needs time to safely release all that absorbed nitrogen. For deeper insights into preventing DCS, explore the latest Decompression Science research and scientific research on the release of dissolved gases.
Boyle's Law: How Pressure Changes Cause Barotrauma
While Henry's Law explains dissolved gases, Boyle's Law handles the immediate mechanical effects of pressure changes on air spaces in your body. This law is beautifully simple: when pressure increases, gas volume decreases, and vice versa.
Your body contains several air-filled spaces that Boyle's Law affects directly - your lungs, ears, sinuses, and even the air space in your mask. As you descend, increasing pressure compresses these air spaces, potentially causing painful "squeezes" if you don't equalize properly.
Ear squeeze happens when pressure outside your eardrum exceeds the pressure inside, pushing your eardrum inward painfully. Sinus squeeze creates similar problems in your sinus cavities. These issues are usually preventable through proper equalization techniques.
But here's where Boyle's Law becomes truly dangerous: lung overexpansion during ascent. If you hold your breath while ascending, the air trapped in your lungs expands as external pressure decreases. From just 33 feet deep, that air would try to double in volume.
Your lungs can't handle this rapid expansion. The delicate air sacs rupture, causing barotrauma that can lead to collapsed lungs or, most dangerously, Arterial Gas Embolism (AGE). In AGE, air bubbles from torn lung tissue enter your bloodstream and travel to your brain or other vital organs, blocking blood flow.
This is why diving's golden rule exists: "Never hold your breath!" Always exhale continuously during ascent. It's such a fundamental safety principle that it's drilled into every new diver from day one.
Understanding pressure-related injuries is crucial for every diver. Learn more about managing these conditions in our guide on Evaluation and Management of Pain-Related Medical Problems of Diving.
Dalton's Law: Why Partial Pressures Are a Key Part of Which Gas Law Best Explains Diving Medical Problems
Dalton's Law brings us into the sophisticated world of gas mixtures and partial pressures. This law states that each gas in a mixture behaves as if it's alone, contributing its own pressure to the total.
Why does this matter underwater? Because the effects of any gas depend on its partial pressure, not just its percentage in your breathing mix. As you descend and pressure increases, the partial pressure of each gas increases proportionally.
Take normal air - roughly 79% nitrogen and 21% oxygen. At the surface, oxygen's partial pressure is 0.21 atmospheres. At 100 feet (4 atmospheres), that oxygen partial pressure jumps to 0.84 atmospheres. Nitrogen goes from 0.79 to 3.16 atmospheres.
These liftd partial pressures create two major problems:
Nitrogen narcosis kicks in when nitrogen's partial pressure rises above about 3 atmospheres, typically around 100 feet on air. You'll feel like you've had a few drinks - impaired judgment, reduced coordination, and sometimes euphoria or anxiety. Divers call it "rapture of the deep," but there's nothing romantic about making poor decisions underwater.
Oxygen toxicity becomes dangerous when oxygen's partial pressure exceeds 1.4 atmospheres for extended periods. Too much oxygen under pressure can cause seizures, which underwater usually means drowning. Recreational diving limits generally keep oxygen partial pressure below 1.4 atmospheres for safety.
Smart divers use alternative gas mixtures to manage these risks. Nitrox contains more oxygen and less nitrogen than air, reducing nitrogen narcosis and extending bottom times. Trimix adds helium to the mix, allowing much deeper dives by further reducing narcotic effects.
Understanding where and why decompression problems occur helps divers make informed decisions. Explore more about this topic in our article Why and at what sites decompression sickness can occur.
How Dive Computers Apply These Laws for Your Safety
Modern dive computers are like having a physics professor strapped to your wrist, constantly calculating how these gas laws affect your body. These remarkable devices use sophisticated decompression algorithms to track gas tension and tissue saturation in real-time.
Your dive computer measures depth and time, then applies Henry's Law to estimate how much nitrogen is dissolving into various theoretical tissue compartments in your body. It considers Boyle's Law effects on pressure changes and uses Dalton's Law to account for different gas mixtures.
The computer displays crucial safety information: no-decompression limits (NDLs) showing how long you can stay at depth, required decompression stops if you exceed those limits, and recommended safety stops to help prevent bubble formation.
These devices have revolutionized diving safety by allowing flexible dive profiles that adapt to your actual dive, not just pre-planned tables. However, computers use mathematical models - they're incredibly useful tools, but individual physiology varies.
Always follow your computer's guidance, ascend slowly, and never ignore symptoms of diving medical problems. When using alternative breathing gases like nitrox or trimix, ensure your computer is properly configured for your gas mixture.
The beauty of understanding these gas laws isn't just academic - it's practical knowledge that keeps you safe and improves every dive you make.
Applying the Science: Key Takeaways for Safer Diving
Now that we've explored how Henry's Law, Boyle's Law, and Dalton's Law each contribute to our understanding of which gas law best explains diving medical problems, it's time to put this knowledge to work. While Henry's Law often takes center stage for decompression sickness, the interplay of all three laws reveals the true complexity of what happens to our bodies underwater.
Think of these gas laws as your invisible dive buddies - they're always there, working behind the scenes. The good news? Once you understand their basic principles, applying them becomes second nature.
Never hold your breath - this golden rule comes straight from Boyle's Law. When you ascend, that air in your lungs wants to expand. Let it out by exhaling continuously, and you'll prevent serious lung overexpansion injuries and arterial gas embolism. It's that simple, yet that critical.
Slow and steady wins the race when it comes to ascending. Henry's Law teaches us that dissolved gases need time to leave our tissues safely. A controlled ascent - typically no faster than 30 feet per minute - gives nitrogen the chance to off-gas gradually through your lungs instead of forming dangerous bubbles. Good buoyancy control makes this much easier to achieve.
Planning isn't just paperwork - it's prevention in action. When you stick to no-decompression limits and perform safety stops, you're directly applying Henry's Law to prevent decompression sickness. Your dive computer does the math, but you make the smart choices.
Know what you're breathing becomes crucial as you advance in diving. Dalton's Law reminds us that partial pressures matter more than percentages. Whether you're breathing air, nitrox, or trimix, understanding your gas mixture helps you avoid oxygen toxicity and manage nitrogen narcosis at depth.
Training never stops being important. Dr. Michael B. Strauss emphasizes in his diving research that knowledge truly is your best dive buddy. Proper training from certified instructors gives you the foundation, but staying current keeps you sharp. The underwater world is amazing, but it demands respect and understanding.
Your dive computer is smart, but you're smarter when you understand what it's telling you. These devices continuously apply all three gas laws to keep you safe, calculating tissue saturation and decompression requirements in real-time. Trust the technology, but never stop thinking for yourself.
Listen to your body - it's often the first to signal when something's wrong. Early recognition of potential diving medical problems can make all the difference. Whether it's joint pain suggesting decompression sickness or confusion hinting at nitrogen narcosis, your body provides valuable feedback.
By respecting these unseen forces and understanding how which gas law best explains diving medical problems applies to real diving situations, you can explore the underwater world with confidence and safety. The physics might be invisible, but their effects are very real - and very preventable when you dive smart.
For more comprehensive insights into diving safety and medical considerations, explore the extensive resources on Diving Science.

Starting on a scuba diving trip can be one of the most thrilling experiences of your life. To ensure each dive is as safe as it is unforgettable, it's crucial to continue your education. Dr. Michael B. Strauss, a renowned expert in diving safety, offers invaluable insights in his comprehensive book, "Diving Science, 5th Ed." An essential read for both novices and experienced divers, you can get your copy here: https://www.bestpub.com/view-all-products/product/diving-science-revisited/category_pathway-48.html
DISCLAIMER: Articles are for "EDUCATIONAL PURPOSES ONLY", not to be considered advice or recommendations.






